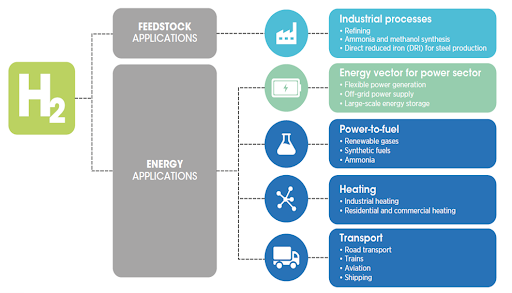
General Technical Questions
A water molecule is made up of two hydrogen atoms and one oxygen atom. Hence the name H2O.
Water electrolysis is the process of using electricity to split water into hydrogen and oxygen.
Electrolysis takes place within a unit known as an electrolyser. An electrolyser consists of a negatively charged cathode and a positively charged anode, separated by an electrolyte.
The electrolyte can be solid metal material such as zirconium as in the case of Polymer Exchange Membrane electrolysers.
The electrolyte can also be a liquid alkaline solution of potassium or sodium hydroxide as in the case of Alkaline electrolysers.
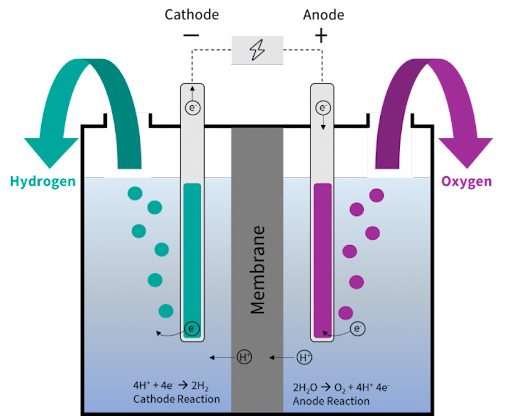
The process of electrolysis:
- Water reacts at the anode to form oxygen and positively charged hydrogen ions (protons).
- The electrons flow through an external circuit and the hydrogen ions selectively move across the PEM to the cathode.
- At the cathode, hydrogen ions combine with electrons from the external circuit to form hydrogen gas.
- Anode Reaction: 2H2O → O2 + 4H+ + 4e-
- Cathode Reaction: 4H+ + 4e- → 2H2
Renewable energy is energy derived from natural sources that are replenished at a higher rate than they are consumed. Such as solar, wind, geothermal, hydro and biomass.
- Hydrogen is the simplest, most abundant element in the universe.
- It is very light and has the lowest molecular weight of all elements.
- It is odourless, colourless and tasteless. It is highly permeable, flammable and easily ignited.
- Hydrogen has a high energy content by weight but the energy density per volume is low at standard pressure and temperature.
- This has a direct impact for storage and would require hydrogen to be compressed and stored under pressure or converted to cryogenic liquid hydrogen.
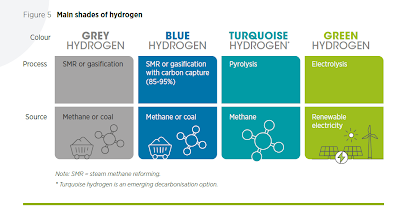
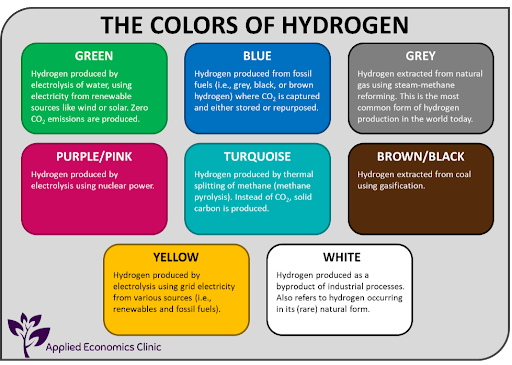
There is no universally accepted colour spectrum for hydrogen and even up to a rainbow of nine colours have been used to describe hydrogen.
IRENA has simplified the different types of hydrogen as follows:
Grey Hydrogen: Is hydrogen produced through the Steam Methane Reforming (SMR) of natural gas or through coal gasification. Coal gasification is the most carbon intensive method of producing hydrogen and brown/black hydrogen is often used to describe hydrogen produced through this process.
Blue Hydrogen: Is hydrogen produced through SMR of natural gas or coal gasification, but the carbon dioxide that is produced is captured and stored or processed for industrial use (carbon capture utilization and storage). CCUS however, does not result in all of the carbon dioxide being captured.
Turquoise Hydrogen: Is hydrogen extracted by using the thermal splitting of methane via methane pyrolysis. This is an emerging field and has not yet been proven on an industrial level.
Green Hydrogen: Is hydrogen produced via the electrolysis of water using renewable energy sources.
As with all fuels, there is a level of danger associated with hydrogen.
By nature hydrogen is non-toxic. Also, hydrogen is very light as compared to air which would allow it to dissipate easily.
By understanding the properties of hydrogen, specific engineering controls and procedures can be implemented to ensure its safe use and to minimise the risks.
| PROPERTIES | DESIGN IMPLICATIONS |
| Hydrogen is very light and has the lowest molecular weight of all chemical species |
– Hydrogen will disperse easily when released – Low molecular weight can impact performance of rotating machinery – Material selection needs to cater for hydrogen embrittlement potential |
| Hydrogen is odorless, colorless and tasteless |
– Difficult for humans to detect – Automated detection methods must be employed – Adequate ventilation required |
| Hydrogen is highly flammable and easily ignited |
– Adequate separation from ignition sources – Fire protection measures must consider the potential for re-ignition and explosion |
| Hydrogen burns with a high temperature, nearly invisible flame |
– Difficult to detect fire – Due diligence must be applied in the selection of fire and gas detection devices |
Hydrogen production via water electrolysis is a proven mature technology, around for approximately 100 years. However, the electrolysers produced and in operation are on the small scale and not in the MW range.
NewGen Project Technical Questions
- The NewGen Project involves the development of a Hydrogen Production Facility to produce hydrogen through the electrolysis of water using renewable and energy-efficient power sources.
- Raw water for the electrolysis will be supplied from the nation’s public utility company, Water & Sewerage Authority (WASA).
- Electricity will be supplied from another public utility company, Trinidad & Tobago Electricity Commission (T&TEC) grid network, via redundant 220kV, 60Hz transmission lines.
- Power to the facility will be allocated from a combination of green power from the Lightsourcebp solar power facility, and carbon neutral power from converting simple cycle machines at PowerGen to combined cycle.
Trinidad and Tobago is the largest industrial base in the Caribbean and is one of the world’s largest exporters of ammonia and methanol. Consequently, Trinidad & Tobago is ranked within the top 5 in CO2 emissions per capita in the world.
There is currently a shortage of natural gas to supply the downstream petrochemical industry. Additionally, the cost of natural gas production has escalated in Trinidad recently.
The hydrogen produced from the NewGen facility increases the availability of feedstock, while simultaneously providing a greener input compared to hydrogen produced via steam reforming of natural gas.
- Serves as a catalyst to decarbonize the energy sector and accelerate the transition to a hydrogen economy
- Assists in decarbonizing the petrochemical sector and secures its viability by providing a greener hydrogen feedstock
- Results in reduced GHGs as ammonia is produced through a greener feedstock
- Helps T&T achieve Paris agreement targets
- Resolidifies T&T position as a leader in the energy sector
- Efficiency gains
This project requires 130MW of power which will be allocated from a combination of renewable energy power and energy-efficient power.
- Renewable energy from new Lighsourcebp solar facilities at Brechin Castle and Orange Grove
- Energy-efficient power by retrofitting existing simple cycle machines at PowerGen Pt Lisas to combined cycle machines where waste heat which is currently vented to the atmosphere is captured and used for additional power generation with negligible increase in GHGs
The power required for the project will be produced for the project and therefore would not negatively impact the nation’s energy supply to existing residential, commercial and industrial customers.
Approximately 160,000 gpd of water is required for the electrolysis process which will be supplied from WASA. This accounts for approximately 0.1% of the nation’s water supply.
However, there will be a consequent reduction in the water demand at the ammonia facility as hydrogen produced through the steam methane reforming process will be displaced by hydrogen produced through electrolysis.
Essentially, there will be a net zero increase in overall water demand.
The NewGen facility will not cause the release of greenhouse gas emissions from the production of hydrogen via electrolysis.
The product carbon footprint for the facility is directly influenced by the carbon intensity of the power sources. The project will utilize renewable power from the Lightsourcebp solar facility and energy-efficient power by retrofitting existing simple cycle machines to combined cycle. As the solar facility will result in zero emissions, the product carbon footprint for the NewGen facility will be determined by the incremental gas consumption associated with the operation of combined cycle machines, which is negligible.
The NewGen project will result in an average reduction of 165,000 metric tonnes Co2 per year.